
Nidel & Nace - Blog

Nidel & Nace Begins Investigation into Sludge Disposal in Central Florida
Join us as we delve into the environmental and health impacts of sewage sludge disposal at the Albritton Biosolids Application Site. Fill out the form below for more information. Nidel & Nace, PLLC is actively investigating claims related to groundwater contamination,...

Justice for Curtis Bay Residents Following Coal Plant Explosion
On Thursday, December 30, 2021 residents of Curtis Bay, Maryland felt their homes shake as a loud boom echoed throughout their community. When residents looked outside, they saw a plume of black smoke rising from the coal terminal to the east of their neighborhood....
Nidel & Nace investigating soil and groundwater contamination in Bethel Eugene, OR
"Although the levels of napthalene exceed health guidelines, these guidelines are designed to be health protective and levels just above the guidelines are not likely to result in adverse health effects." Oregon Health AuthorityThe town of Bethel in Eugene, Oregon...
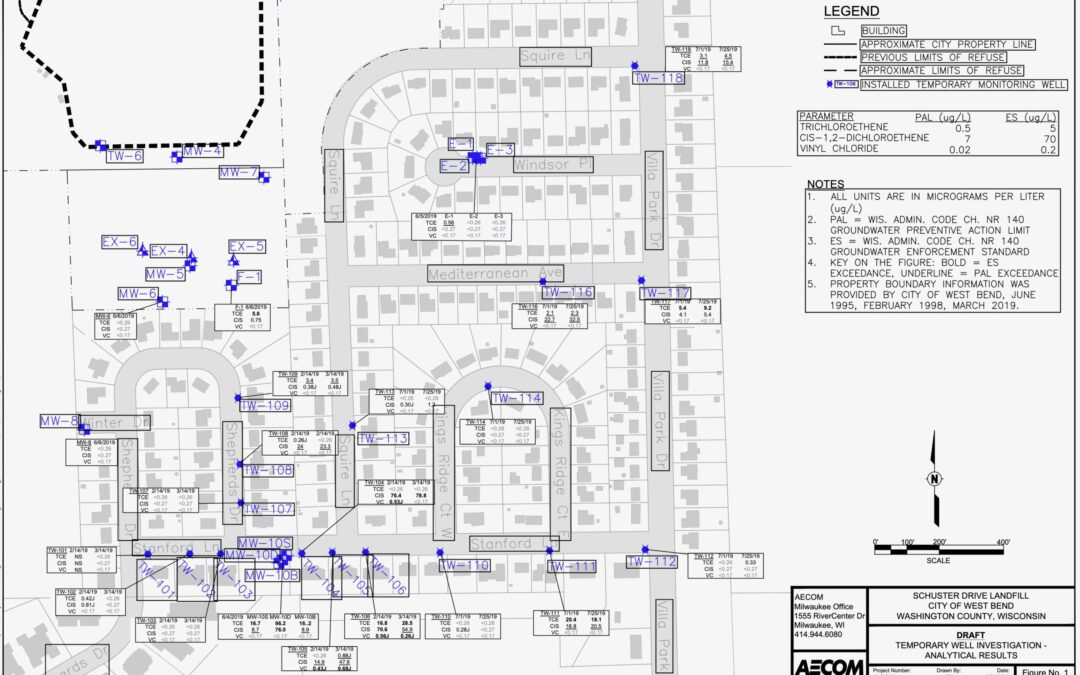
Update on Schuster Landfill
Page providing updates on the Schuster Drive landfill and its impact on the residents of Villa Park in Wes

Class Action Filed Against Toxic Bethel Wood Treatment Plant
"Although the levels of napthalene exceed health guidelines, these guidelines are designed to be health protective and levels just above the guidelines are not likely to result in adverse health effects." Oregon Health Authority The town of Bethel has shared its air...
SSRI Use During Pregnancy May Damage Human Brain
A group of researchers, including those from the John's Hopkins Bloomberg School of Public Health, found that the molecule paroxetine (the active ingredient in the antidepressant Paxil and one of the class of SSRI antidepressants) is associated with developmental...
Deadly Refinery Pollution in Artesia, New Mexico
The HollyFrontier refinery in Artesia, New Mexico has not been a good neighbor to those living in the immediate vicinity. According to this analysis by the Environmental Integrity Project, fence line benzene levels far exceed the safe limits established by the...
Liberty University Students Return Amid Pandemic Raising Liability for Liberty
As the White House extends social distancing guidelines amide the coronavirus pandemic until at least April 30th of this year, students in Virginia are returning to classes at Liberty University. This despite the State of Virginia and Govern Ralph Northam's order...
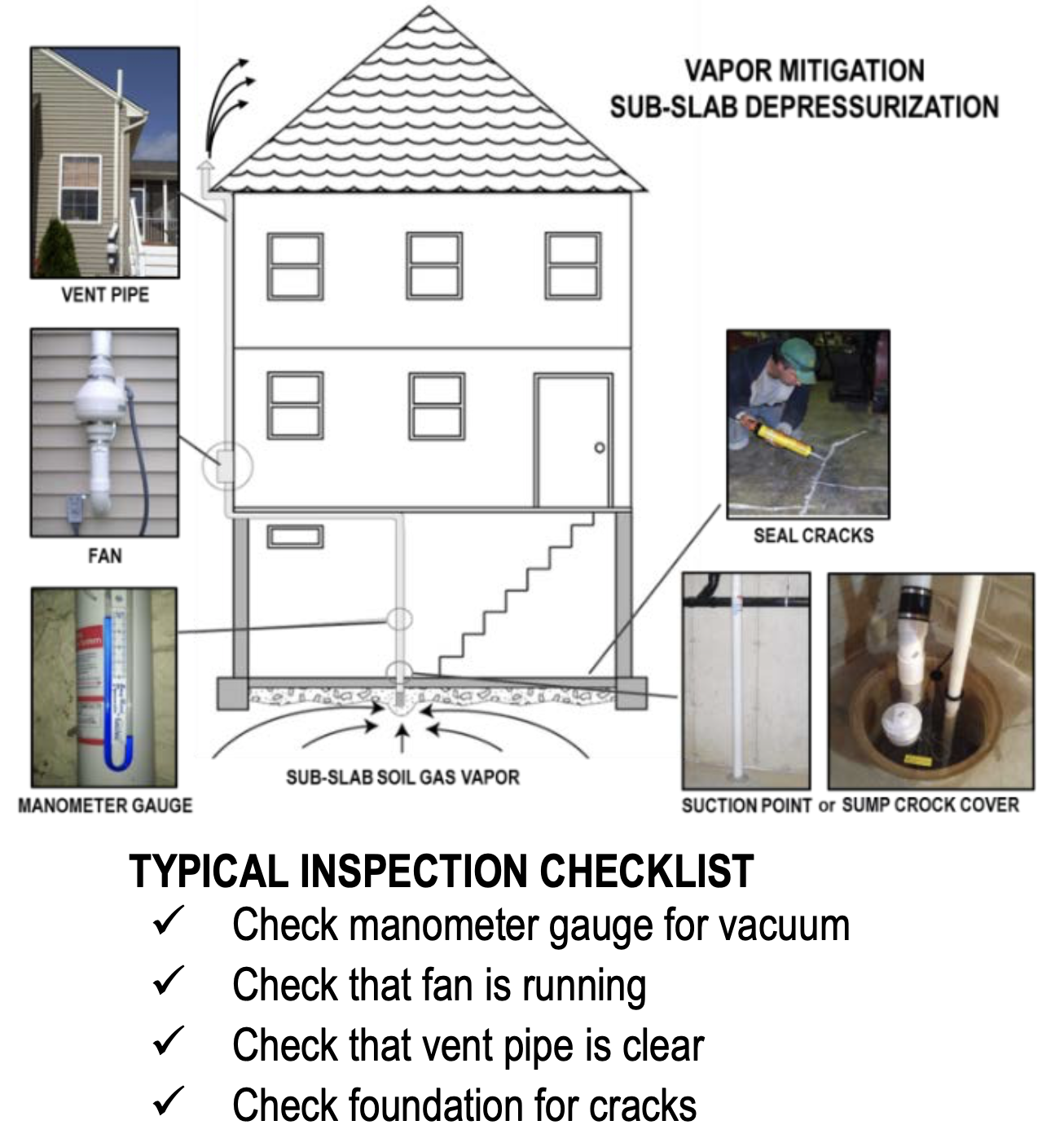
Legacy Landfill Poses a Chemical Threat to Villa Park Subdivision
Residents in the Villa Parks subdivision have reached out to our firm with concerns about the presence of toxic chemicals in and around their homes. These concerns range from concerns about the health and wellbeing of family members to the impact that the newly...
Nidel & Nace Pursues Class Action at Enclave Apartments
Tenants at the Enclave Apartments in Silver Spring, Maryland have been living in unsafe and dangerous conditions due to the presence of mold, infestation and unhygienic conditions. They have now filed a Class Action Complaint against the current and former building...